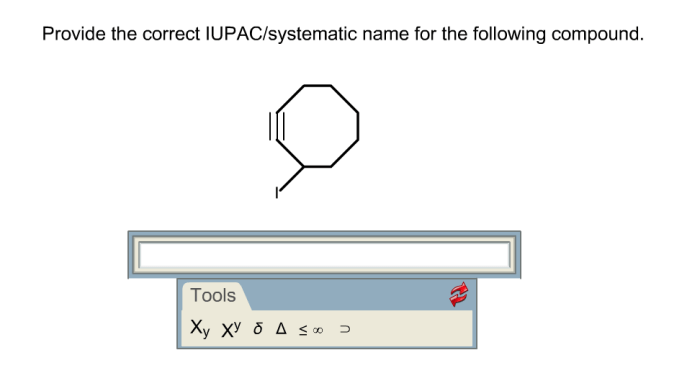
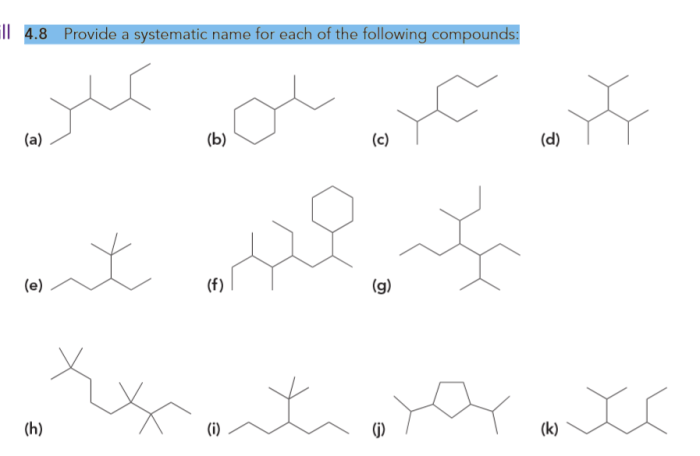
Give the systematic names of these compounds. spelling counts. – Give the systematic names of these compounds: spelling counts. This topic is a fundamental aspect of chemistry, providing a standardized language for naming and identifying chemical compounds. Understanding systematic nomenclature is crucial for effective communication, safety, and accuracy in the field of chemistry.
This guide will delve into the principles and rules of systematic nomenclature, empowering you to confidently assign precise and unambiguous names to various chemical compounds. By mastering this skill, you will enhance your ability to navigate the intricate world of chemistry with clarity and precision.
Systematic Nomenclature of Organic Compounds: Give The Systematic Names Of These Compounds. Spelling Counts.
Systematic nomenclature is a set of rules used to name organic compounds in a consistent and unambiguous manner. It is based on the principles of IUPAC (International Union of Pure and Applied Chemistry) and allows chemists to communicate the structure and properties of compounds clearly and accurately.
The purpose of systematic nomenclature is to provide a unique and universally recognized name for each organic compound. This is important for several reasons. First, it allows chemists to identify and discuss compounds without confusion. Second, it helps to organize and classify compounds based on their structure and properties.
Third, it facilitates the retrieval of information about compounds from databases and literature sources.
Functional Group Identification, Give the systematic names of these compounds. spelling counts.
The first step in systematic nomenclature is to identify the functional group(s) present in the compound. A functional group is a specific arrangement of atoms that imparts characteristic chemical properties to the compound. The most common functional groups include alkanes, alkenes, alkynes, alcohols, aldehydes, ketones, carboxylic acids, and amines.
IUPAC has established rules for naming functional groups. These rules are based on the type of atoms present in the functional group and the number of bonds between the atoms. For example, alkanes are named by adding the suffix “-ane” to the root name of the parent chain.
Alkenes are named by adding the suffix “-ene” to the root name of the parent chain. Alkynes are named by adding the suffix “-yne” to the root name of the parent chain.
Parent Chain Selection
Once the functional group(s) have been identified, the next step is to select the parent chain. The parent chain is the longest continuous chain of carbon atoms in the compound. If there are multiple chains of equal length, the chain with the most functional groups is chosen as the parent chain.
The criteria for selecting the parent chain are as follows:
- The parent chain must contain the functional group.
- The parent chain must be the longest continuous chain of carbon atoms.
- The parent chain must have the most functional groups.
Numbering the Parent Chain
Once the parent chain has been selected, the next step is to number the carbon atoms in the chain. The carbon atoms are numbered from one end of the chain to the other, starting with the carbon atom that is closest to the functional group.
The rules for numbering the parent chain are as follows:
- The carbon atom that is closest to the functional group is assigned the number 1.
- The carbon atoms are numbered consecutively from one end of the chain to the other.
- If there are multiple functional groups, the carbon atom that is closest to the highest priority functional group is assigned the number 1.
Naming Substituents
The next step is to name the substituents that are attached to the parent chain. Substituents are any atoms or groups of atoms that are not part of the parent chain. Substituents are named by adding the appropriate prefix to the root name of the substituent.
The prefix indicates the number of carbon atoms in the substituent.
The IUPAC rules for naming substituents are as follows:
- The prefix for a substituent with one carbon atom is “methyl-“.
- The prefix for a substituent with two carbon atoms is “ethyl-“.
- The prefix for a substituent with three carbon atoms is “propyl-“.
- The prefix for a substituent with four carbon atoms is “butyl-“.
- The prefix for a substituent with five carbon atoms is “pentyl-“.
- The prefix for a substituent with six carbon atoms is “hexyl-“.
Putting It All Together
Once the functional group, parent chain, numbering, and substituents have been identified, the systematic name of the compound can be assembled. The systematic name is written in the following order:
- The prefix for the substituents.
- The root name of the parent chain.
- The suffix for the functional group.
For example, the systematic name of the compound CH3CH2CH2OH is 1-propanol. The prefix “1-” indicates that the hydroxyl group is attached to the first carbon atom of the parent chain. The root name “propan-” indicates that the parent chain is a three-carbon chain.
The suffix “-ol” indicates that the functional group is an alcohol.
Top FAQs
What is the purpose of systematic nomenclature?
Systematic nomenclature provides a standardized and unambiguous system for naming chemical compounds, ensuring clear communication and accurate identification among chemists.
How do I determine the parent chain in a compound?
The parent chain is the longest continuous chain of carbon atoms in the compound. If there are multiple chains of equal length, the one with the most branches or functional groups takes precedence.
What are the rules for numbering the parent chain?
The parent chain is numbered from one end to the other, giving the lowest possible numbers to the substituents. Double or triple bonds are treated as single bonds for the purpose of numbering.