Based on relative bond strengths classify these reactions as endothermic – Based on relative bond strengths, we can classify reactions as endothermic. Understanding this relationship provides valuable insights into chemical processes, with applications in various fields. Delving into the concept of endothermic reactions, we explore the intricate interplay between bond strength and enthalpy change.
Endothermic reactions absorb energy from their surroundings, leading to an increase in enthalpy. This energy absorption often results in the breaking of strong bonds and the formation of weaker bonds. By examining the relative bond strengths of reactants and products, we can determine whether a reaction is endothermic or exothermic.
Relative Bond Strengths and Endothermic Reactions: Based On Relative Bond Strengths Classify These Reactions As Endothermic
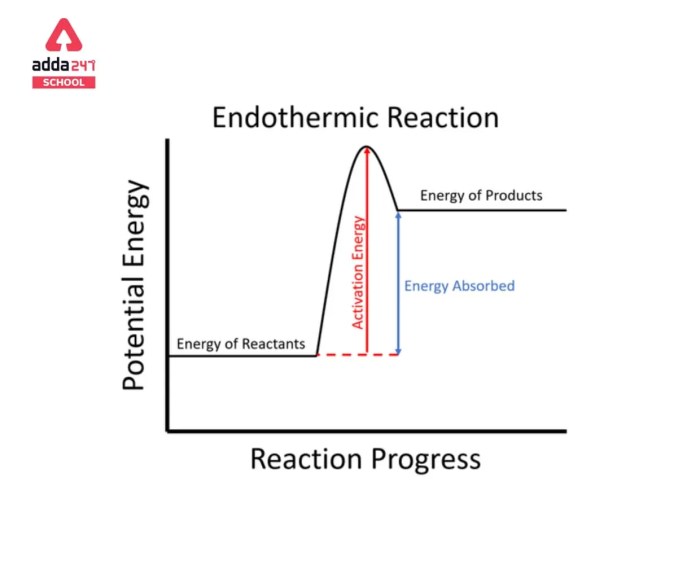
Endothermic reactions are chemical reactions that absorb energy from their surroundings. The energy absorbed is used to break bonds in the reactants, and the products have stronger bonds than the reactants. The enthalpy change of an endothermic reaction is positive, indicating that the products have more energy than the reactants.
The relative bond strengths of the reactants and products can be used to predict whether a reaction is endothermic or exothermic. If the products have stronger bonds than the reactants, the reaction is endothermic. If the products have weaker bonds than the reactants, the reaction is exothermic.
Bond Strength and Enthalpy
The bond strength is the amount of energy required to break a bond. The stronger the bond, the more energy is required to break it. The enthalpy change of a reaction is the difference in energy between the products and the reactants.
A positive enthalpy change indicates that the products have more energy than the reactants, and a negative enthalpy change indicates that the products have less energy than the reactants.
The bond strength and enthalpy change of a reaction are related. If the products have stronger bonds than the reactants, the enthalpy change of the reaction will be positive. If the products have weaker bonds than the reactants, the enthalpy change of the reaction will be negative.
Classification of Reactions
Reactions can be classified as endothermic or exothermic based on their relative bond strengths. The following table summarizes the different types of reactions and their corresponding bond strength changes:
| Type of Reaction | Bond Strength Change | Enthalpy Change |
|---|---|---|
| Endothermic | Products have stronger bonds than reactants | Positive |
| Exothermic | Products have weaker bonds than reactants | Negative |
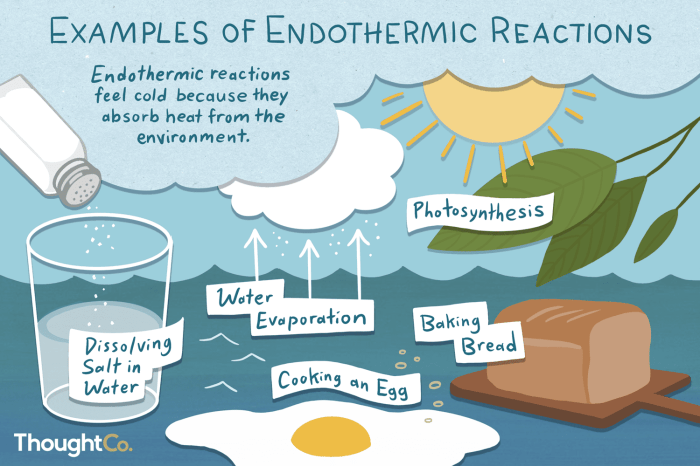
Here are some examples of endothermic reactions:
- The melting of ice
- The boiling of water
- The sublimation of dry ice
Applications, Based on relative bond strengths classify these reactions as endothermic
The understanding of endothermic reactions has many practical applications. For example, endothermic reactions are used in:
- The cooling of food and drinks
- The production of chemicals
- The development of new materials
For example, the cooling of food and drinks is an endothermic process. When you put a cold drink in the refrigerator, the heat from the drink is transferred to the refrigerator, causing the drink to cool down. The refrigerator uses an endothermic reaction to remove the heat from the drink.
FAQ Section
What is the significance of bond strength in endothermic reactions?
Bond strength plays a crucial role in determining whether a reaction is endothermic or exothermic. Endothermic reactions involve the breaking of strong bonds and the formation of weaker bonds, leading to an overall increase in enthalpy.
How can we predict the endothermic nature of a reaction based on bond strengths?
By comparing the bond strengths of reactants and products, we can determine the enthalpy change of a reaction. If the enthalpy change is positive, the reaction is endothermic, indicating the absorption of energy from the surroundings.